Mary Elizabeth Hartnett

Professor of Ophthalmology & Visual Sciences
Adjunct Professor of Pediatrics
Adjunct Professor of Neurobiology
Neurobiology of Disease
e-mail: ME.Hartnett@hsc.utah.edu
B.S. 1982, Rensselaer Polytechnic Institute; M.D. 1983, Albany Medical College; Research
Fellow, 1987-1989, Schepens Eye Research Institute, Harvard Medical School
RESEARCH:
Mechanisms of vascular genesis, dysgenesis and reorganization in retina
Mary Elizabeth Hartnett, M.D. is the Principal Investigator of an NIH-funded laboratory that studies mechanisms of normal and aberrant angiogenesis, particularly related to diabetic retinopathy, retinopathy of prematurity and age-related macular degeneration. The mission of the laboratory is to understand what causes blood vessels to grow outside their normal tissue compartments and into other areas of the eye where they cause damage. Rather than inhibit or destroy abnormal vessels, the goal is to understand what simulates endothelial cells of blood vessels to become activated to migrate and proliferate aberrantly, and once this is known, to then restore or contain blood vessel support to normal ocular compartments.
Dr. Hartnett and her team of researchers are investigating multiple causes of aberrant angiogenesis. One is retinal avascularity, or a lack of blood vessel support in areas of the inner retina that leads to retinal hypoxia, which stimulates aberrant growth of blood vessels. One important observation the Hartnett lab made was that overactivation of the signaling pathway of vascular endothelial growth factor (VEGF) actually contributes to retinal avascularity by disordering the growth of endothelial cells, causing them to grow into the gel of the eye (vitreous) as intravitreal neovascularization (IVNV, Fig 1, showing a retina flat-mount stained with lectin to visualize the vasculature) rather than normally into the retina. Another cause of abnormal angiogenesis is the generation of damaging reactive oxygen species, which can lead to loss of the integrity of the cell junctions, which are important in maintaining normal compartmentalization of tissues.
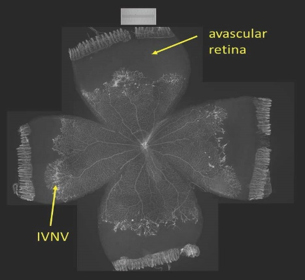
Fig. 1
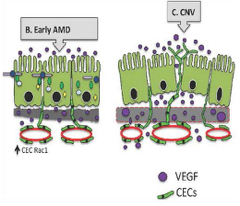
Fig. 2
The Hartnett lab uses transgenic mouse models or models in other species that better represent human diseases. In species in which transgenic modeling is difficult, Dr. Hartnett has used gene therapy to knockdown proteins in specific cells in the retina. These techniques have been important in understanding cell-specific roles of proteins within the retina and also in understanding the interactions that different cells within the retina have on one another to lead to pathology as opposed to what is learned from solo cell experiments.
To study mechanisms of invasive choroidal neovascularization in age-related macular degeneration (AMD), a co-culture model is used to understand causes of endothelial migration across the retinal pigment epithelium (Fig 2). This involves cell-cell models, animal models and study of human tissue. Relevant stresses associated with human AMD are used to test activation of signaling pathways compromising the integrity of the retinal pigment epithelial barrier and/or activating endothelial cells to migrate.
The Hartnett Laboratory uses a number of imaging methods such as the Micron IV in combination with fluorescein angiography and spectral domain optical coherence tomography to analyze retinal structure and to localize laser injury simultaneously to regions within the retina that have been transduced by gene therapy. These methods, in conjunction with cell coculture techniques, help the lab work out mechanisms of disease with the hopes of finding cures for blinding eye diseases.
Dr. Hartnett’s LAB impact in Moran Eye Center Patients
The Hartnett laboratory targets questions in order to reduce blindness and improve or preserve vision in patients with eye diseases. The Hartnett laboratory uses a systematic approach to understand physiologic (normal) and pathologic processes with the goal to seek homeostasis and restore physiology, or normalcy. The focus has been on angiogenesis, or blood vessel growth. Blood vessels provide oxygen and nutrition to the retina and eye and these are critical for normal eye function and vision. However, many blinding eye diseases are associated with abnormal blood vessel growth. The stimulus for this abnormal growth is many times first a loss of the normal blood vessels, followed by abnormal blood vessel growth. Current treatment focus on anti-angiogenic strategies. If blood vessel growth is inhibited through anti-angiogenic treatments, then the stimulus is not removed and abnormal blood vessels may grow again. In addition, some of the substances implicated in abnormal blood vessel growth also have beneficial effects on the eye. The Hartnett laboratory seeks to understand the mechanisms to inhibit pathologic aspects of these substances, and by so doing redirect blood vessel growth into normal compartments and/or prevent invasion of blood vessel growth into unwanted areas of the eye, rather than broadly inhibit blood vessel growth.
